Sarah barely noticed the slight tenderness in her gums during her routine dental cleaning. The hygienist mentioned something about “pockets of inflammation,” but Sarah was already thinking about her afternoon meetings. She promised to floss more regularly, just like she had promised last year and the year before that.
Twenty years later, Sarah’s morning coffee ritual changed forever. Her right hand trembled as she lifted the mug, and her once-fluid handwriting had become cramped and shaky. The neurologist’s diagnosis hit like a thunderbolt: early-stage Parkinson’s disease. No family history. No obvious risk factors. Just a mystery that left her asking “Why me?”
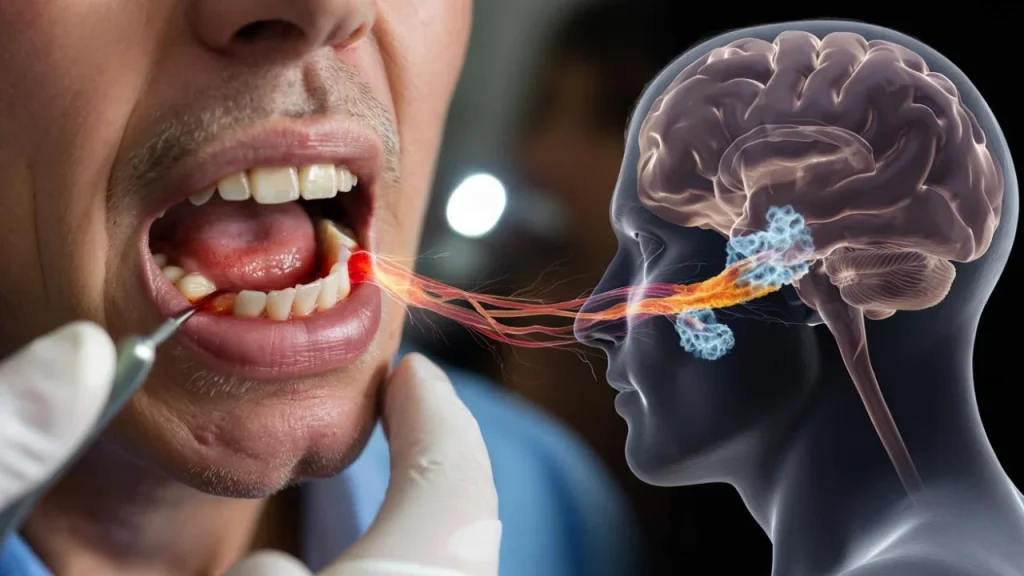
Now, groundbreaking research suggests the answer might have been hiding in plain sight, right in her mouth all along. Scientists are uncovering a shocking connection between Parkinson’s disease and a common mouth bacterium that silently damages the brain decades before any symptoms appear.
The Hidden Highway From Gums to Brain
Parkinson’s disease has always seemed like a cruel lottery of genetics and aging. But researchers are painting a dramatically different picture—one where the disease’s roots trace back to something as mundane as bleeding gums and poor dental hygiene.
- The hidden reason your body reacts like it’s in crisis when nothing bad has happened
- Landlord enters tenant’s garden to harvest fruit, sparking heated debate over rental property rights
- AI rocket propulsion breakthrough could cut Mars travel time in half
- Deep-Sea Military Operation Uncovers Unprecedented Archaeological Discovery at Record Depth
- China artificial islands: 12-year sand dumping project reshapes entire ocean territories
- The Endless Cycle: Why Setting Clear Boundaries is Essential for Effective Cleaning
The culprit is Porphyromonas gingivalis, a notorious bacterium that thrives in the spaces between your teeth and gums. This isn’t just any harmless mouth bug. It’s a sophisticated pathogen that releases toxic enzymes capable of traveling through your bloodstream and crossing into your brain tissue.
“We’re seeing clear evidence that oral bacteria can establish themselves in brain tissue and trigger the inflammatory cascades that characterize Parkinson’s,” explains Dr. Michael Chen, a neuroinflammation researcher at Stanford University. “The mouth-brain connection is far more direct than we ever imagined.”
Recent studies have found traces of Parkinson’s mouth bacteria and their inflammatory byproducts in brain tissue samples from deceased Parkinson’s patients. Even more alarming, large-scale population studies consistently show that people with severe, chronic gum disease face significantly higher risks of developing neurodegenerative diseases later in life.
The process unfolds over decades. Chronic inflammation in the gums creates tiny tears in tissue, allowing bacteria to slip into the bloodstream. Once there, these microscopic invaders can breach the blood-brain barrier and begin their destructive work, triggering the protein clumping and cell death that define Parkinson’s disease.
The Science Behind the Mouth-Brain Connection
Understanding how Parkinson’s mouth bacteria cause brain damage requires looking at the inflammatory cascade they trigger. When P. gingivalis establishes itself in brain tissue, it doesn’t just cause direct damage—it sets off a chain reaction that can persist for years.
Here’s what researchers have discovered about this process:
- Bacterial toxins promote misfolding of alpha-synuclein proteins, the hallmark of Parkinson’s disease
- Chronic inflammation damages dopamine-producing neurons in the substantia nigra
- Immune system overactivation creates a self-perpetuating cycle of brain cell death
- Bacterial enzymes called gingipains directly attack brain proteins essential for neural function
- The inflammatory response can begin 15-20 years before motor symptoms appear
Perhaps most striking is the timeline. Brain changes associated with Parkinson’s mouth bacteria exposure can be detected decades before the first tremor or balance problem emerges.
| Study Type | Key Finding | Risk Increase |
|---|---|---|
| Brain tissue analysis | P. gingivalis DNA found in 7 of 10 Parkinson’s brains | N/A |
| Population study (50,000 people) | Severe gum disease linked to neurodegeneration | 38% higher risk |
| Longitudinal tracking | Chronic periodontitis predicts Parkinson’s onset | 28% increased likelihood |
| Mouse model research | Oral P. gingivalis infection causes brain protein clumping | 6x more alpha-synuclein |
“The data is becoming impossible to ignore,” notes Dr. Jennifer Rodriguez, a periodontal disease specialist at UCLA. “We’re seeing consistent patterns across multiple study designs and populations. Poor oral health isn’t just about cavities anymore—it’s a potential brain health issue.”
Who’s Really at Risk and What Changes Now
This research completely reframes who should worry about Parkinson’s disease. Instead of focusing solely on age and genetics, we need to consider oral health history as a major risk factor.
The highest-risk individuals include:
- Adults over 40 with untreated gum disease lasting more than 5 years
- People who’ve lost multiple teeth due to periodontal disease
- Individuals with chronic inflammation markers in routine blood work
- Those with a history of avoiding dental care or irregular cleanings
- Smokers and diabetics, who face higher risks of both gum disease and inflammation
The implications extend far beyond individual health decisions. Healthcare systems may need to integrate dental and neurological care in unprecedented ways. Insurance companies might start covering more aggressive periodontal treatments as preventive medicine.
“We’re looking at a paradigm shift where your dentist becomes part of your Parkinson’s prevention team,” explains Dr. Amanda Foster, a neurologist specializing in movement disorders. “Regular cleanings and gum disease treatment might be some of our most powerful tools for protecting brain health.”
Early intervention strategies are already being developed. Some clinics now screen Parkinson’s patients for oral bacteria and recommend intensive periodontal therapy alongside traditional treatments. Researchers are even testing whether eliminating P. gingivalis from the mouth can slow disease progression in early-stage patients.
For millions of people currently walking around with bleeding gums and “just a little tartar,” this research represents both a wake-up call and an opportunity. Unlike genetic risk factors that can’t be changed, oral health is completely within our control.
The mouth-brain connection also opens new therapeutic possibilities. If Parkinson’s mouth bacteria can trigger disease, then targeting these bacteria might prevent or slow progression. Clinical trials are underway testing everything from specialized antimicrobial treatments to vaccines designed to eliminate P. gingivalis.
But perhaps the most powerful message is simpler: that daily flossing and six-month cleanings might be protecting far more than just your smile. They could be safeguarding your future ability to write, walk, and live independently.
FAQs
Can treating gum disease prevent Parkinson’s disease?
While research is ongoing, eliminating chronic gum inflammation appears to reduce neurodegeneration risk significantly. Early treatment may be key.
How long does it take for mouth bacteria to affect the brain?
Studies suggest the inflammatory process can begin within months of bacterial colonization, but symptom development typically takes 15-20 years.
Is everyone with gum disease at risk for Parkinson’s?
Not everyone with gum disease will develop Parkinson’s, but severe, long-term periodontal disease does increase risk substantially compared to healthy gums.
Can antibiotics eliminate the risk?
Simple antibiotics may temporarily reduce bacterial levels, but comprehensive periodontal therapy and ongoing oral hygiene are more effective long-term strategies.
Should I get tested for P. gingivalis bacteria?
Some dental offices now offer bacterial testing, though comprehensive gum disease treatment addresses multiple harmful bacteria simultaneously and may be more practical.
Is this research definitive?
While the evidence is compelling and growing stronger, researchers emphasize that Parkinson’s likely results from multiple factors working together, with oral bacteria being one important piece.